Clinical researchers design and conduct studies to evaluate the safety and efficacy of new treatments, ensuring adherence to ethical standards and regulatory requirements. Clinical coordinators manage day-to-day trial operations, including participant recruitment, data collection, and communication between research teams and sponsors. Both roles are critical to successful clinical trials, with clinical researchers focusing on study design and analysis, while coordinators ensure smooth execution and compliance.
Table of Comparison
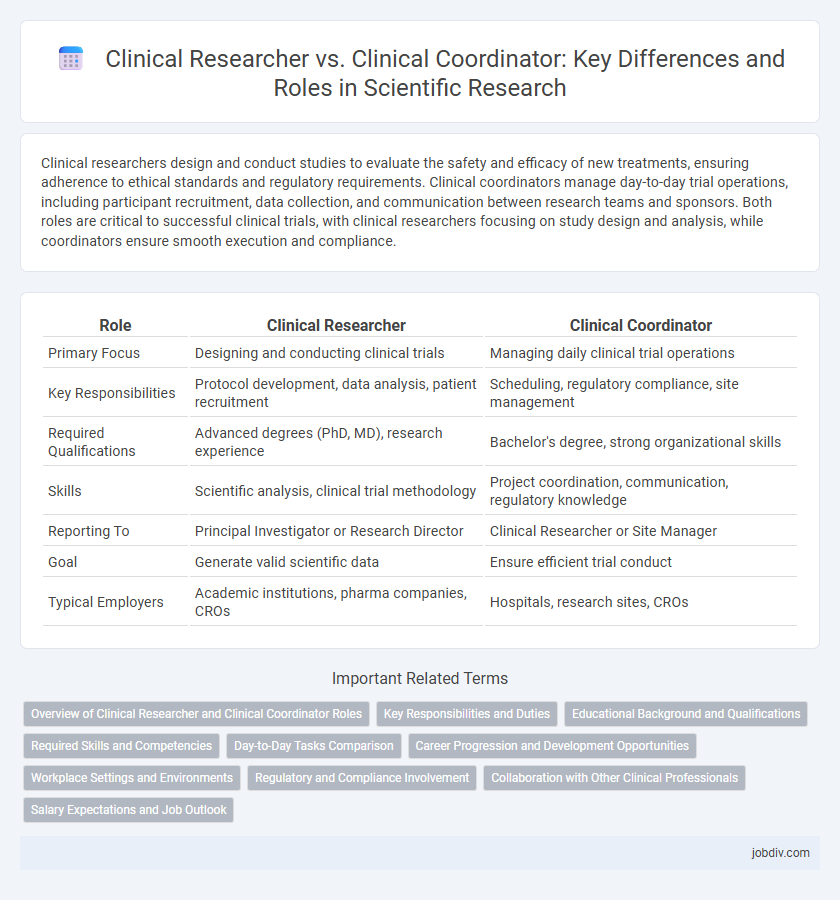
| Role | Clinical Researcher | Clinical Coordinator |
|---|---|---|
| Primary Focus | Designing and conducting clinical trials | Managing daily clinical trial operations |
| Key Responsibilities | Protocol development, data analysis, patient recruitment | Scheduling, regulatory compliance, site management |
| Required Qualifications | Advanced degrees (PhD, MD), research experience | Bachelor's degree, strong organizational skills |
| Skills | Scientific analysis, clinical trial methodology | Project coordination, communication, regulatory knowledge |
| Reporting To | Principal Investigator or Research Director | Clinical Researcher or Site Manager |
| Goal | Generate valid scientific data | Ensure efficient trial conduct |
| Typical Employers | Academic institutions, pharma companies, CROs | Hospitals, research sites, CROs |
Overview of Clinical Researcher and Clinical Coordinator Roles
Clinical researchers design and oversee clinical trials, ensuring the scientific integrity and regulatory compliance of study protocols to generate reliable data on medical interventions. Clinical coordinators manage daily trial operations, including patient recruitment, data collection, and adherence to study timelines, facilitating smooth workflow between researchers and participants. Both roles are critical in advancing clinical studies, with researchers concentrating on study design and data analysis, while coordinators focus on operational execution and participant management.
Key Responsibilities and Duties
Clinical Researchers design and conduct clinical trials, focusing on protocol development, patient recruitment, data collection, and analysis to evaluate new treatments' safety and efficacy. Clinical Coordinators manage the daily operations of clinical studies, ensuring compliance with regulatory requirements, coordinating between research teams, and maintaining accurate documentation. While Clinical Researchers drive scientific inquiry and data interpretation, Clinical Coordinators facilitate study logistics and regulatory adherence to support trial success.
Educational Background and Qualifications
Clinical Researchers typically possess advanced degrees such as a Master's or PhD in fields like Clinical Research, Life Sciences, or Public Health, emphasizing strong research methodology and biostatistics skills. Clinical Coordinators often hold a Bachelor's degree in Nursing, Health Administration, or related areas, with certifications like CCRP (Certified Clinical Research Professional) enhancing their qualifications for overseeing trial operations. Both roles require comprehensive knowledge of Good Clinical Practice (GCP) guidelines and regulatory compliance, ensuring ethical and effective clinical trial conduct.
Required Skills and Competencies
Clinical researchers require strong analytical skills, proficiency in study design, and expertise in data interpretation to ensure valid and reliable outcomes. Clinical coordinators must possess exceptional organizational abilities, adeptness in regulatory compliance, and effective communication skills to manage trial logistics and coordinate team activities efficiently. Both roles demand knowledge of Good Clinical Practice (GCP), attention to detail, and the ability to adapt to evolving protocols in clinical research environments.
Day-to-Day Tasks Comparison
Clinical Researchers primarily design studies, analyze data, and ensure protocol adherence to advance medical knowledge, while Clinical Coordinators manage patient recruitment, schedule visits, and oversee regulatory documentation on a daily basis. Clinical Researchers spend significant time interpreting trial outcomes and liaising with scientific teams, whereas Clinical Coordinators focus on logistics, patient communication, and maintaining compliance with trial timelines. Both roles require coordination but differ in scope, with Researchers oriented toward study development and analysis, and Coordinators emphasizing operational execution.
Career Progression and Development Opportunities
Clinical researchers typically engage in designing and conducting studies, gaining expertise in protocol development and data analysis, which can lead to advanced roles such as Principal Investigator or Clinical Project Manager. Clinical coordinators focus on managing day-to-day trial operations, patient recruitment, and regulatory compliance, building skills that facilitate progression to Senior Coordinator or Clinical Trial Manager positions. Both roles offer distinct career development paths with opportunities to specialize in therapeutic areas or transition into broader clinical operations leadership.
Workplace Settings and Environments
Clinical researchers primarily operate in academic institutions, pharmaceutical companies, and research hospitals where they design and oversee clinical trials, ensuring protocol compliance and data integrity. Clinical coordinators typically work within hospital research units or clinical trial sites, managing day-to-day trial operations, patient recruitment, and regulatory documentation. Both roles require collaboration with multidisciplinary teams but are distinguished by their focus on trial design versus trial management within healthcare and research environments.
Regulatory and Compliance Involvement
Clinical Researchers primarily design and oversee clinical trials, ensuring adherence to regulatory standards such as FDA guidelines and ICH-GCP principles. Clinical Coordinators manage the day-to-day operations of trials, maintaining compliance by monitoring informed consent processes and accurate documentation according to institutional review boards (IRB) requirements. Both roles are critical in upholding ethical standards and regulatory compliance throughout the clinical research lifecycle.
Collaboration with Other Clinical Professionals
Clinical researchers collaborate closely with clinical coordinators to design and implement study protocols, ensuring accurate data collection and patient safety. Clinical coordinators manage day-to-day trial operations, facilitating communication between research teams, healthcare providers, and regulatory bodies. Effective collaboration between these roles enhances trial efficiency, compliance, and overall research outcomes.
Salary Expectations and Job Outlook
Clinical researchers typically command higher salaries, with average annual earnings ranging from $70,000 to $110,000, reflecting their advanced responsibilities in designing and conducting trials. Clinical coordinators earn between $50,000 and $80,000 per year, managing day-to-day operations and ensuring protocol compliance. Job outlook for clinical researchers shows a growth rate of 7% through 2030, driven by increased clinical trial activity, while clinical coordinators are expected to grow at 6%, supported by expanding healthcare infrastructure and multidisciplinary team collaboration.
Clinical Researcher vs Clinical Coordinator Infographic
 jobdiv.com
jobdiv.com