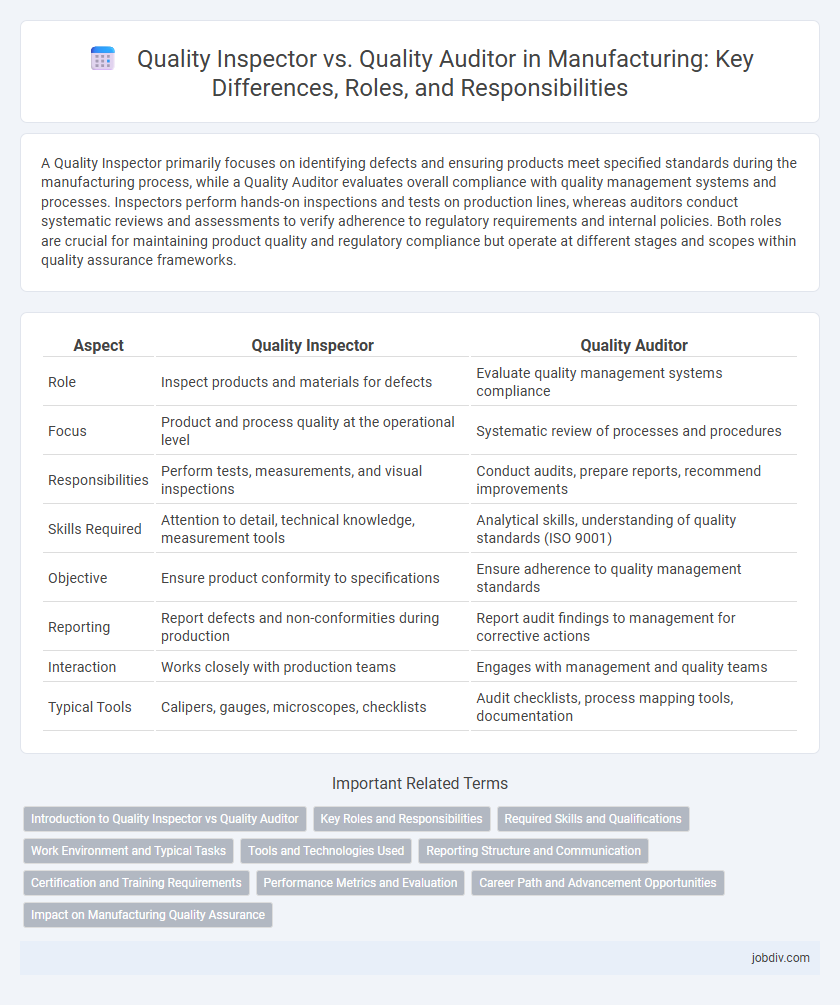
A Quality Inspector primarily focuses on identifying defects and ensuring products meet specified standards during the manufacturing process, while a Quality Auditor evaluates overall compliance with quality management systems and processes. Inspectors perform hands-on inspections and tests on production lines, whereas auditors conduct systematic reviews and assessments to verify adherence to regulatory requirements and internal policies. Both roles are crucial for maintaining product quality and regulatory compliance but operate at different stages and scopes within quality assurance frameworks.
Table of Comparison
| Aspect | Quality Inspector | Quality Auditor |
|---|---|---|
| Role | Inspect products and materials for defects | Evaluate quality management systems compliance |
| Focus | Product and process quality at the operational level | Systematic review of processes and procedures |
| Responsibilities | Perform tests, measurements, and visual inspections | Conduct audits, prepare reports, recommend improvements |
| Skills Required | Attention to detail, technical knowledge, measurement tools | Analytical skills, understanding of quality standards (ISO 9001) |
| Objective | Ensure product conformity to specifications | Ensure adherence to quality management standards |
| Reporting | Report defects and non-conformities during production | Report audit findings to management for corrective actions |
| Interaction | Works closely with production teams | Engages with management and quality teams |
| Typical Tools | Calipers, gauges, microscopes, checklists | Audit checklists, process mapping tools, documentation |
Introduction to Quality Inspector vs Quality Auditor
Quality Inspectors perform hands-on evaluations of manufacturing processes and products to ensure compliance with predefined quality standards, using tools such as inspection gauges and test equipment. Quality Auditors systematically review process documentation, conduct audits, and verify adherence to quality management systems like ISO 9001, focusing on process effectiveness and regulatory compliance. Both roles are critical in manufacturing quality assurance, but Inspectors emphasize product-level checks while Auditors oversee process integrity and continuous improvement.
Key Roles and Responsibilities
Quality Inspectors primarily conduct hands-on examinations of products at various stages of the manufacturing process to ensure compliance with specified standards and detect defects. Quality Auditors systematically review and assess quality management systems, processes, and documentation to verify adherence to regulatory requirements and internal policies. Both roles are essential for maintaining product integrity, with inspectors focused on operational quality control and auditors emphasizing process evaluation and continuous improvement.
Required Skills and Qualifications
Quality Inspectors require strong attention to detail, proficiency in measurement tools, and knowledge of manufacturing standards such as ISO 9001, alongside technical skills in reading blueprints and conducting visual inspections. Quality Auditors need advanced analytical skills, expertise in audit procedures, regulatory compliance, and comprehensive knowledge of quality management systems to assess process adherence and implement corrective actions. Certifications such as Certified Quality Inspector (CQI) for inspectors and Certified Quality Auditor (CQA) for auditors enhance credibility and demonstrate proficiency in these specialized roles.
Work Environment and Typical Tasks
Quality Inspectors operate primarily on the production floor, conducting hands-on inspections and monitoring manufacturing processes to ensure products meet specifications. Quality Auditors work in diverse environments including offices and production sites, systematically reviewing quality management systems, documentation, and compliance with regulatory standards. Typical tasks for Quality Inspectors involve defect detection, measurement verification, and reporting, whereas Quality Auditors focus on auditing processes, identifying non-conformances, and recommending corrective actions.
Tools and Technologies Used
Quality Inspectors utilize tools such as calibrated measuring instruments, visual inspection equipment, and handheld scanners to perform on-site product evaluations and ensure compliance with specifications. Quality Auditors rely on advanced data analysis software, audit management systems, and digital checklists to systematically review processes, standards adherence, and quality documentation. Both roles integrate technologies like statistical process control (SPC) software and enterprise resource planning (ERP) systems to enhance accuracy and efficiency in manufacturing quality assurance.
Reporting Structure and Communication
Quality Inspectors typically report to Production Managers or Quality Control Supervisors, ensuring immediate feedback on manufacturing defects and process deviations. Quality Auditors generally report to Quality Assurance Managers or Compliance Officers, providing comprehensive evaluations and audit findings for strategic decision-making. Communication for Inspectors is often direct and operational, while Auditors maintain formal, documented dialogues with multiple departments and senior management.
Certification and Training Requirements
Quality Inspectors typically require certification such as ASQ Certified Quality Inspector (CQI) or equivalent training focused on product examination and defect identification, emphasizing hands-on skills in manufacturing environments. Quality Auditors often hold certifications like ASQ Certified Quality Auditor (CQA) or ISO 9001 Lead Auditor, reflecting advanced knowledge in auditing principles, regulatory compliance, and process improvement standards. Training for Quality Auditors is more comprehensive, covering audit techniques, quality management systems, and regulatory frameworks, while Quality Inspectors focus on operational standards and inspection methodologies.
Performance Metrics and Evaluation
Quality Inspectors primarily focus on real-time defect detection and process adherence by measuring metrics such as defect rates, inspection accuracy, and cycle times. Quality Auditors evaluate broader compliance and effectiveness through performance indicators like audit scores, corrective action implementation rates, and process improvement outcomes. Both roles contribute to manufacturing excellence but differ in scope, with Inspectors emphasizing operational quality control and Auditors targeting systemic process evaluations.
Career Path and Advancement Opportunities
Quality Inspectors typically start their careers by conducting hands-on product and process inspections, gaining practical experience in detecting defects and ensuring compliance with specifications. Quality Auditors often advance from inspection roles by developing expertise in audit methodologies, risk assessment, and regulatory standards, positioning themselves for higher-level responsibilities such as quality management and process improvement leadership. Career advancement for Quality Auditors generally offers broader strategic influence within manufacturing organizations, including roles in quality systems development and regulatory compliance management.
Impact on Manufacturing Quality Assurance
Quality Inspectors conduct hands-on evaluations of products and processes to identify defects and ensure compliance with manufacturing specifications, directly preventing faulty outputs from reaching customers. Quality Auditors perform systematic reviews and assessments of quality management systems, verifying adherence to standards such as ISO 9001 and driving continuous improvement initiatives. Together, these roles enhance manufacturing quality assurance by combining real-time defect detection with strategic process validation, promoting consistent product reliability and regulatory compliance.
Quality Inspector vs Quality Auditor Infographic
 jobdiv.com
jobdiv.com